
Synthetic spike-in standards improve run-specific systematic error analysis for DNA and RNA sequencing. DNA damage is a pervasive cause of sequencing errors, directly confounding variant identification. Sequencing depth and coverage: key considerations in genomic analyses. Reference materials and reference measurement procedures: an overview from a national metrology institute.
#NGS SEQUENCE ANALYSIS ISO#
ISO Guide 30:2015 - Reference Materials - Selected Terms and Definitions (ISO, 2015).īunk, D. International Organization for Standardization. Impact of reference materials on accuracy in clinical chemistry. Roadmap for harmonization of clinical laboratory measurement procedures. Developing a sustainable process to provide quality control materials for genetic testing. Good laboratory practices for molecular genetic testing for heritable diseases and conditions. The Nex-StoCT (Next-generation Sequencing: Standardization of Clinical Testing) workgroup developed a set of guidelines to ensure that results from NGS tests are sufficiently reliable for clinical diagnosis, including the recommendation of reference standards for test validation, quality control and proficiency testing.Ĭenters for Disease Control and Prevention. Assuring the quality of next-generation sequencing in clinical laboratory practice. Opportunities and challenges associated with clinical diagnostic genome sequencing. ACMG clinical laboratory standards for next-generation sequencing. College of American Pathologists' laboratory standards for next-generation sequencing clinical tests. Good laboratory practice for clinical next-generation sequencing informatics pipelines. Assuring the quality of next-generation sequencing in clinical microbiology and public health laboratories. Guidelines for diagnostic next-generation sequencing. Systematic evaluation of Sanger validation of next-generation sequencing variants. Sanger confirmation is required to achieve optimal sensitivity and specificity in next-generation sequencing panel testing. Library preparation methods for next-generation sequencing: tone down the bias. This study investigated the location of clinically relevant variants in regions of the human genome that are refractory to reliable genotyping with NGS owing to the presence of extreme GC content or repetitive sequences. Medical implications of technical accuracy in genome sequencing. Next-generation sequencing for infectious disease diagnosis and management. Translating RNA sequencing into clinical diagnostics: opportunities and challenges. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. Furthermore, reference standards will have a key role in developing the next generation of sequencing technologies. We consider an informed use of reference standards, along with associated statistical principles, to be essential for the rigorous analysis of NGS data. Each approach has its own strengths and limitations.ĭespite recent progress in developing reference standards, several important challenges remain, including the need to establish the commutability of standards with patient samples.
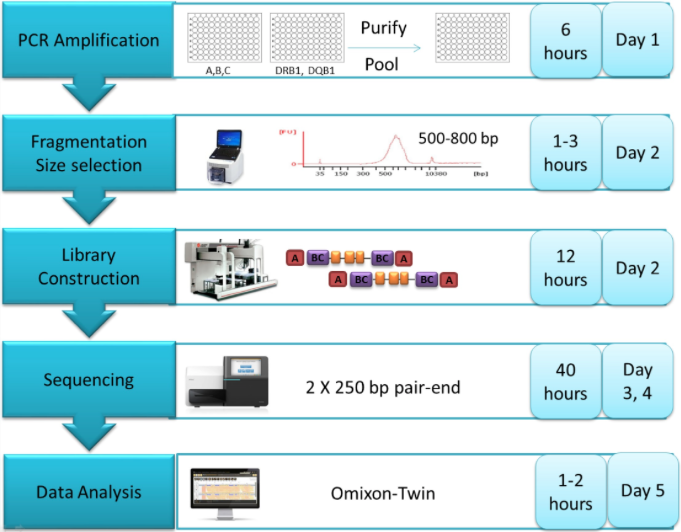
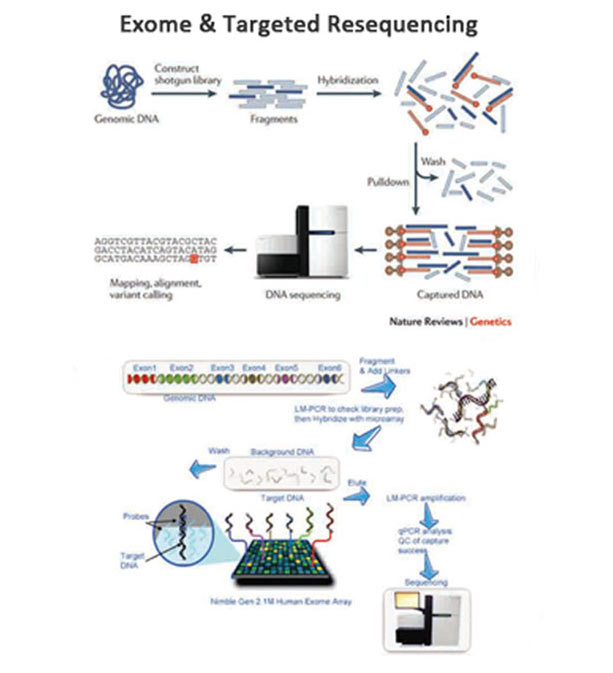
Various reference standards have been developed for NGS, including well-characterized biological samples, synthetic controls and in silico data sets. These errors and biases can be mitigated through the use of reference standards - materials with known characteristics that are crucial for test development, quality control and proficiency testing. In a clinical context, this can lead to false positives and false negatives, and the potential for misdiagnosis. The analysis of next-generation sequencing (NGS) data is complex, owing to the breadth of sequences tested and the range of internal biases and errors.


 0 kommentar(er)
0 kommentar(er)
